Lecture notes for Monday, March 16, 2015
Tissues and organs that develop from mesoderm
| Lateral plate mesoderm |
Intermediate mesoderm |
Somites | Notochord | Somites | Intermediate mesoderm |
Lateral plate mesoderm |


|
We are members of the phylum "chordata", all of the members of which have a notochord at some stage of their development. In mammals and birds, the function of the notochord is replaced by the vertebral column (bones of the back-bone+intervertebral discs) But tadpoles and larval fish actually depend on the notochord to allow them to swim by alternating contraction of muscles along each side.
The notochord mesoderm also induces formation of the neural tube. The structure of the notochord is a long stack of (not always) flattened cells, that often have very much the geometry of a stack of coins. Wrapped tightly around the surface of this long cylinder of cells are layers of fibers of type I collagen in many spiraling layers, that alternate in direction (a clockwise layer, then a counterclockwise layer, then another clockwise layer, etc. etc. etc.) The cells inside the notochord then form large vacuoles in their cytoplasm, which makes each cell swell in volume, pushing against the spiral layers of (strong & non-stretchable!) collagen fibers. The result is that the notochord can bend side to side, but can't be compressed along its anterior-posterior axis.
Tail of a living tadpole. The broad line just below the center
Neural tube and notochord
Notochord of salamander
Section through chick embryo
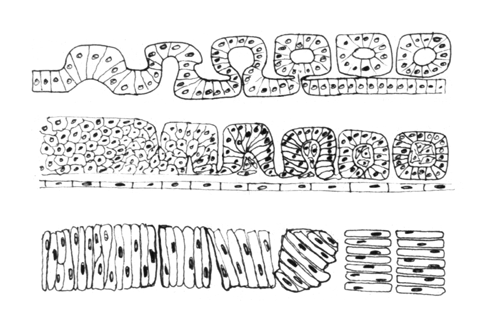
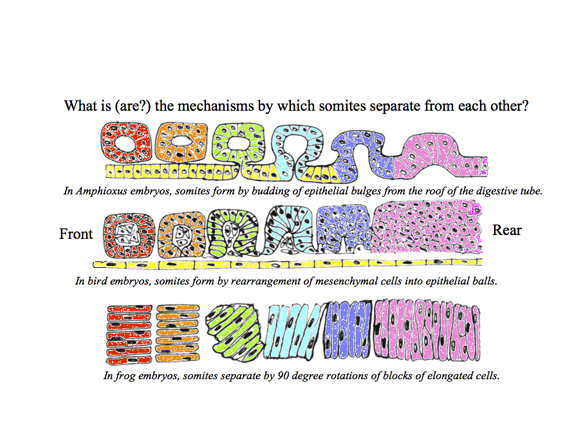
************************************************* Somites are segmental blocks that form beside the neural tube. At first, there are continuous columns of "paraxial" mesoderm
The drawing above shows the geometrical rearrangements that some scientists have reported to casue somite segmentation in different classes of chordates. Top: Amphioxus, Middle: bird and mammal, Bottom: frogs. Anterior is to right side.
Then these columns spontaneously split apart, one pair at a time.
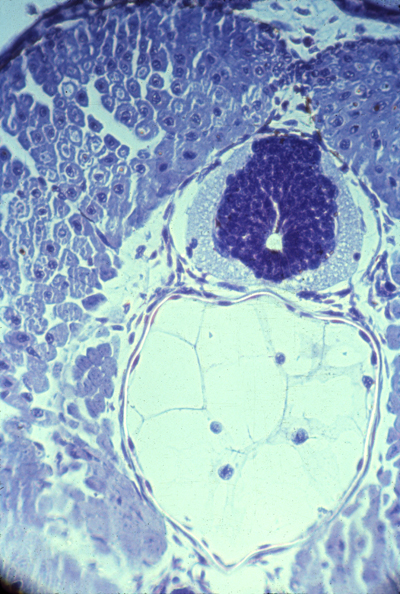
Longitudinal section of notochord and somites in a developing frog embryo
Somites forming in a fixed whole mount chicken embryo. Anterior to left.
Somites forming in a living chicken embryo. Anterior to left.
The physical mechanism of splitting apart has now been discovered to be stimulation of decreased cell-cell adhesions by specific binding of the proteins "Ephrin" and "Eph." These stimulate cells to break their adhesions to each other, and guide optic nerve fibers to their map-like patterns of connections in the roof of the mid-brain. Some textbooks say that Ephrins cause cell to repel each other, but really they induce detachment of adhesions; Cell traction pulls each side in a tug of war, therefore weakening of cell adhesions causes them to crawl directionally away from the weakened adhesions. These names are acronyms for Erythropoietin secreting Hepatocyte, because they were first discovered as secretions of a kind of liver cancer cells. Many important proteins got discovered because some kind of cancer cells secreted them. The Fibroblast growth factors and NGF (Nerve Growth Factor) both got discovered because certain cancer cells secreted them. Rita Levi-Montalcini shared a Nobel Prize for discovering NGF. In scanning EM photos, "somitomeres" can be seen, where a somite is going to form, apparently as the first stages of somite formation. In most species, somites start as hollow epithelial balls, and conversion of cells from being mesenchymal to epithelial therefore seems to be part or the process of separation. (These epithelial cells seem to have basal surfaces outward) The "Clock-and-Wavefront Hypothesis" was invented by Cooke and Zeeman to explain somite formation, and especially the ability of embryos to adjust somite size in proportion to the amount of paraxial mesoderm to be subdivided (If you surgically remove tissue, then smaller somites are made). The theory is/was that one quantity oscillates higher and lower in amount, while another variable forms a gradient that gradually increases in amount along its length. A somite is supposed to be split off each time the oscillator increases. Molecules (mostly proteins) have been discovered to behave as the theory predicted. But there are surprises! Three gradients have been found: Fibroblast Growth Factor, Wnt and Retinoic acid (the RA gradient running in the opposite direction [anterior high to posterior low] to the others). Fish use different "clock" substances than birds & mammals (Notch protein) Arthropods also use clocks & wavefronts; which wasn't expected.
For most species, the same number of somites forms in each embryo. You form as many vertebrae as your embryo had formed pairs of somites.
Each somite subdivides into four parts: The dermatome --> cells form the inner layer of skin (dermis) The myotome ---> all the skeletal muscle cells of the body The anterior sclerotome --> the posterior half of a vertebra! The posterior sclerotome --> the anterior half of a vertebra!
Not a typographical error! Each somite gives cells to 2 vertebrae
That way muscles run from one vertebra to the next,
Somites only temporarily exist in the embryo: Incidentally, to Neurologists a "dermatome" means the stripe-shaped areas of skin and muscles that are innervated by sensory and motor nerves from each segmental spinal nerve. In the disease "Shingles", stripe-shaped zones of blisters form on the skin (looking like shingles on a roof) because rows of blisters form on the skin of different dermatomes. Neurologists have a method for mapping paralysis caused by slipped disks, by inserting electrodes under the skin and firing one set of nerves at a time. This method hurts quite a bit.
|
 |
Three somites Dermatomes farthest to the left Myotomes right under them Then sclerotomes The continuous blue stripe on the right is the neural tube | |
 |
Myotomes in salamander | |
 |
Polarization microscopy shows that myotomes develop muscle fibers very early | |
 |
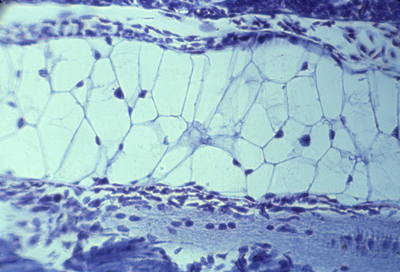
Alternating anterior and posterior sclerotomes | |
| Nerve ganglia form only in the anterior sclerotomes. Also notice that small arteries run just below (behind?) each of these ganglia. Although somites somehow cause the locations of these nerve ganglia and the arteries branching from the aorta, neither the nerves or arteries develop from somite cells. Surgical transplantations of salamander somites (5 from the tail replacing 3 from the trunk) prove that anatomical segmentation is caused by somites themselves, NOT as additional effects of what-ever signals cause somites to form.
|
||
 |
Longitudinal section of 3 somites, that goes right through the myotome | |
| Notice the bundles of nerve fibers located just to the right (toward the midline of the embryo) of each of these three myotomes. |
|
*************************************************
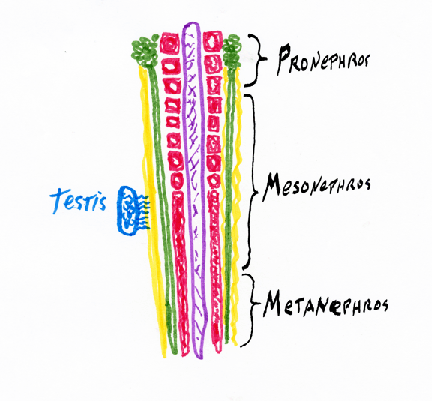
Intermediate mesoderm differentiates to form
kidneys, kidney ducts, and male sex ducts. The most anterior intermediate mesoderm forms the pronephros ("head kidney") (one on each side)
Pronephros
From each pronephros extends a pronephric duct.
Intermediate mesoderm tubules
In amphibia all the rest of the intermediate mesoderm differentiates
into a pair (right & left) of adult kidneys, which use the pronephric duct to carry urine, and to also carry sperm in males.
These adult kidneys are called the metanephros,
and each one has a special duct, called a ureter.
While we are embryos, we use a separate kidney, that differentiates from the middle parts of the intermediate mesoderm.
Lateral plate mesoderm splits into two layers: The female sex ducts (Oviducts; Fallopian tubules, Uterus) differentiate from outfoldings in the coelomic wall.
In Reptiles, Birds and Mammals, *************************************************
The heart also develops from the extreme right and left sides of the lateral plate mesoderm; In reptiles and birds, the lateral plate spreads out across the yolk, & completely surrounding the yolk, so as to form the yolk sac. Embryos of reptiles and birds form their hearts where the right and left lateral plate mesoderm sheets come together, slightly in front of (!) where the head is forming. Later in development, digestion of yolk causes the yolk sac to get progressively smaller, this shifts the location of the heart in a posterior direction, bringing it into the chest of the embryo. If pieces of plastic or other physical barriers were inserted where the heart normally forms, so as to block the right and left sheets of lateral plate mesoderm from coming together !!????
What would you expect would happen?
2) "Half-hearts" develop: incomplete structures on each side? 3) A heart develops only on the left side? 4) A heart develops only on the right side? 5) Two complete, normal hearts develop (one on each side)?
#5) Two complete, normal hearts develop {But they are usually mirror images of each other!} This is one of several examples in which parts of embryos can be kept apart, or mechanically split.
Another example is that if you cut a limb bud in two; Or (also like early embryos) you can graft two early limb buds together, and often it will develop into a single large leg, or wing, etc. These edges of the lateral plate mesoderm can form more than two hearts, when this tissue is cut into pieces. As many as nine separate hearts were formed by one chicken embryo in some experiments done by French embryologists.
The heart has to start pumping blood very early in development, I want to contrast the kidney's solution to this problem of early function in comparison with the solution in the case of the heart. Embryos make several different pairs of kidneys, made out of different parts of the intermediate mesoderm. 3 pairs in mammals, birds & reptiles
Pronephros forming and using the pronephric duct = Wolffian Duct Your adult kidneys are your pair of metanephros kidneys The ureter "grows" (actually its tip crawls) forward from where the Wolffian duct connects to the bladder, and because the ureter induces you most posterior intermediate mesoderm to differentiate into kidney, a few % of people have only one kidney, from which two ureters carry urine to their bladder. If you donate a kidney, check first if you have 2
In males, the Wolffian duct becomes the sperm duct.
In females, the Wolffian duct degenerates (apoptosis??) I don't know!
In contrast, you form one single heart (normally), Imagine re-building a lawn-mower motor into a 4-cycle car engine, while the motor is running full speed the whole time!
Please notice that at the molecular level this is also astonishing,
because the shape changes are driven by special actins and myosins. The heart starts as a simple peristaltic tube, with no valves and no separate chambers. It pulses from rear to front. Later the heart then expands and gradually bends into a U shape. Then its walls pinch inward subdividing into an atrium & ventricle. Then a constriction pulls from front to rear, dividing the heart into right and left atria and ventricles (four chambers) with 4 sets of one-way valves. This increased complexity occurs by modification and addition to the same original tissue, while the heart is beating!
"Intelligent design" might have built several different hearts,
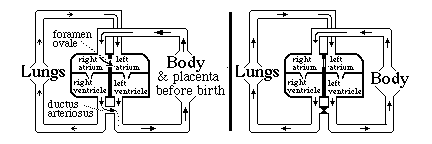
analogous to how the kidney solves the problem of early function. Before birth, blood does not need to be pumped through the lungs. The following system for by-passing the lungs exists: until birth
There is a "trap door" opening between the right and left atria called the foramen ovale,
Without the ductus arteriosus, blood pumped out of the right ventricle would have no choice but to go to the lungs. Without the foramen ovale, only blood returning from the lungs would reach the left atrium. Questions to think about and discuss in class: a) Which direction does blood flow through the foramen ovale, prior to the moment of birth? b) Which direction does blood flow through the ductus arteriosus before birth? c) Why doesn't the blood flow in the other direction in the foramen ovale? d) Why doesn't it flow in the other direction in the ductus arteriosus? When the lungs expand and fill with air, that reduces the mechanical resistance to blood flowing through the capillaries: How should that change the relative pressures in left versus right atria? e) What about the relative amounts of pressure in the aorta versus the pulmonary artery, before and after the lungs expand at birth. f) The thickness and strength of the cardiac muscle walls of the right and left ventricles are the same before birth! But after birth, the muscles of the left wall gradually become about four-times thicker and stronger on the left side than the right side? What does that tell you? Normally (in mammals) the tissues fuse together in both the ductus arteriosus and the foramen ovale, closing both permanently. In as many as 25% of people, some small hole remains between the atria. (without any symptoms) Many kinds of serious birth defects in human hearts result from improper formation of the interventricular septum (septal defects). Sometimes the ventricles remain connected (not separated) Sometimes the aorta or the pulmonary artery are connected to the WRONG ventricle! Such "blue babies" can develop up to the time of birth, more or less "normally", but then have severe problems Surgery can fix most of these problems; but it is one of the most difficult areas of surgery, I have been told.
Circulatory SystemThe special kind of epithelial cells that line all blood vessels are called endothelial cells.The narrowest blood vessels are called capillaries, and their walls are made of endothelial cells only (one cell layer thick; and with few or no smooth muscle cells of fibroblasts to provide mechanical reinforcement or control constriction. Arteries are made of layers of smooth muscle cells (and some fibroblasts) and type I collagen fibers wrapped around their capillary cells. Veins have fibroblasts and collagen wrapping the endothelial tube that lines where the blood cells are. The first beginnings of the vertebrate circulatory system are a series of unconnected endothelial sacks, called blood islands, with red blood cells and white blood cells differentiating inside; most of these are in the yolk sac. Their endothelial cells crawl and connect to each other to form hollow tubes: the first capillaries. Only much later does bone marrow become the place where blood formation occurs; early embryos don't have bones; and when the skeleton does form it is nearly all made out of cartilage, which is solid, without a marrow, and isn't even penetrated by blood vessels (i.e. NOT vascularized) which is what you call it when these endothelial cells penetrate a tissue and form a system of capillaries, and later arteries & veins. Early embryos do not have any bone marrow yet, because nearly all their "bones" are still made of cartilage, and are solid inside. The yolk sac is one of several different organs that are used as locations for the hemopoietic stem cells which form red blood cells (and also white blood cells). These stem cells move to the liver, and later to the bone marrow. Embryonic and fetal hemoglobins are different from (and coded for by different genes than) the hemoglobin we have in the red blood cells we make after birth. Fetal hemoglobin binds oxygen slightly more strongly than adult hemoglobin, which increases the amount of oxygen that is transferred across the placenta from the blood of a pregnant woman to the blood of the fetus developing inside her. The genes for embryonic and fetal hemoglobin proteins are directly adjacent to the genes for adult hemoglobins, with the early blood cells activating one gene, and then later blood cells activating the gene next to that one. The mechanism isn't known, nor is it known whether there is any similarity to the mechanisms that cause adjacent Hox genes to be transcribed in adjacent tissues. A few people continue to make fetal hemoglobin all their life, instead of switching to the adult hemoglobin genes, and the symptoms of this are almost unnoticeable! A new treatment for victims of sickle-cell anemia (and other genetic defects of adult hemoglobins), works by simulating hemopoietic stem cells back to transcribing the genes for fetal hemoglobin. The geometry of the early vertebrate circulatory system is organized much like that of our distant fish ancestors, with blood being pumped front-ward out of the heart and around the sides of the neck in a series of 6 paired arteries like those used to provide blood to the 6 pairs of gills in a fish. Even birds and mammals form these aortic arches, even though there are no actual gills; they are in the places where gills would be. In mammals, the fourth left aortic arch becomes the aorta.
The vertebrate circulatory system begins as a series of unconnected endothelial sacks, called blood islands.
Red blood cells and white blood cells differentiate inside; The yolk sac is one of several different organs that are used as locations for the hemopoietic stem cells which form red blood cells (and also white blood cells). These stem cells move to the liver, and later to the bone marrow.
|