Limb bud development

Cartilages differentiating inside a living chicken hind-limb bud
Before starting discussion of limb buds, we reviewed the topic of electro-osmosis, which was mentioned in the lecture on January 16th. The following notes are repeated from that lecture:
Osmotic swelling is a very important force in shaping embryos.
Cartilage expansion (which is not really "growth") is driven by a special version of osmosis called electroosmosis. This does not depend on semi-permeable membranes, like ordinary osmosis.
Electroosmosis is caused by positive ions (Sodium, potassium, hydrogen, calcium etc.) being held in gels by electric attraction toward negatively charged sulfate groups that are covalently attached to special chains of sugars (chondroitin sulfate, among others). The positive ions are trapped at high concentrations by this electrical attraction, which produces just as much pressure as would result from the same concentration of ions being held inside a semipermeable membrane. (22.4 atmospheres of pressure per one mole concentration of sulfates).
Vertebrate skeletons begin (mostly) as cartilages, much of which gets replaced by bones having the same shapes as the cartilage they replace. It is very important to understand that cartilage never changes into bone, although cartilages often get calcium salts deposited in them.
Except for the roof of the skull, the only way vertebrate embryos can make a bone that has any given shape is to secrete cartilage having that particular shape, and then replace the cartilage with bone.
If you squeeze a piece of cartilage, positive ions get pulled along with water that gets pushed out of cartilage. This creates a brief positive electrical charge, that can be hundreds of volts! (but very low amperage) Then you get a brief voltage in the opposite direction.
Some researchers think these voltages serve some function (rather than just being side effects of the use of electrical attraction as a way to exert and resist pressure). The suspected function is stimulation of bone production. It has definitely been proven that bones get stronger whenever and wherever more forces are exerted on them. For example, tennis players develop much stronger bones in their right arm if they are right-handed.
At a research meeting I went to a couple years ago, people reported that stressed skeletons produced voltages by piezoelectricity, which is a different phenomenon that occurs in many crystals.
Medically, there is an enormous need to discover methods that can produce strengthening of bones. Electric currents are one of the methods that has been tried.
Limb buds develop into legs, arms, wings, or fins.
Normally, embryos form 4 limb buds,
2 anterior & 2 posterior.
But are there ways to induce salamander embryos to form three legs on each side?
(Like the Martians in "John Carter of Mars"!)
Surgically implanting otic or olfactory placodes, or plastic beads containing
Fibroblast Growth Factor, will cause three legs to form on the same side.
Scientists suspect that embryos re-use the same inductive signals in different parts of
the body; and inductively competent tissue is already concentrated along both flanks.
If myotomes are removed, limbs will still form, but they will lack muscles.
Limb buds develop if transplanted to other parts of the body, and to the chorioallantoic membrane of chicken embryos.
You can also split limb buds, fuse limb buds, rotate them upside down, or backwards.
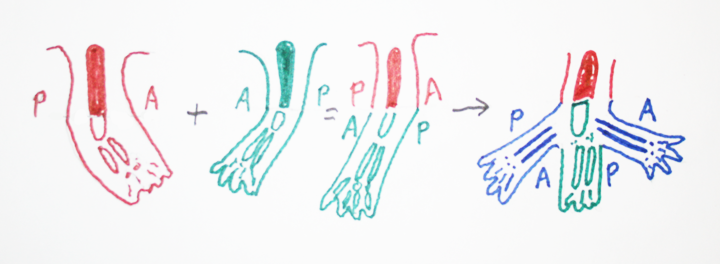
If you cut an early limb bud in two, it can form 2 arms (or legs), or often it will form a branched leg (mirror images).
Fusing two limb buds can often form only one leg.
(Surgically graft one limb bud right next to another one, & they merge!
These kinds of experiments work best in salamander embryos.)
A figure from a textbook showing this kind of experiment was shown in class. We can't repost that because of copyright restrictions, but here is another drawing that shows the same thing:
Notice the similarity to what Driesch discovered! That splitting early echinoderm
embryos in two results in each half developing into a half-sized "scale model" embryo.
Several body organs have been discovered to form two organs, for example two complete hearts.
Another example is eyes, and also the nose rudiment, which can be either split into 2, or two can be fused into one.
The human uterus also forms by side to side fusion of the lower end of the oviduct. (Müllerian Duct).
[Be prepared for an exam question: Describe at least ? embryonic organs that develop by fusion of two masses of cells.
List examples of organs or organisms being split into two, where each half is able to form all parts normally developed
when they are not split.]
Some kinds of parasitic worms often split limb buds in frogs, resulting in 5 or 6 hind limbs!
&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&
Development of the 3 geometric axes are controlled separately in limb buds
And at 3 different times, by 3 different sets of chemical signals
-
Proximo-distal axis
Anterior-posterior axis
Dorso-ventral axis
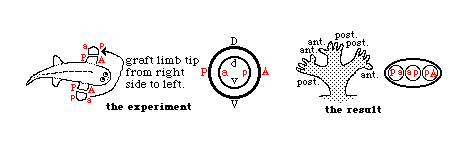
If surgical rotation is done early enough in development,
then regulation occurs, in the sense that
whichever part is
facing toward the front of the embryo will develop into a thumb,
or whatever structure
that species has on the anterior side of its arm, etc.
During a later period of development, limb buds are no longer able to
regulate (change) their anterior-posterior axis,
but can still regulate the dorso-ventral axis!
In effect, that means that the part that would have developed into the palm
of the hand can change its fate,
and develop the geometry appropriate
to the back of the hand, and vice versa,
but a rotated limb bud can no
longer change where the thumb forms, etc.
Analogous surgical experiments were done with the inner ear (otic placode)
cutting it out and grafting it back in,
upside down and backwards, etc.
Before a certain stage, it "regulates" completely, in the sense that the
different cells change how they develop
according to their new orientations,
such that the semi-circular canals, otoliths, etc. all develop at
locations
that are normal with respect to the rest of the body.
At a later stage of development, the dorso-ventral and medio-lateral
axes are still able to regulate,
but the anterior-posterior axis is irreversible.
These rotation experiments were done in salamanders, in the 1910s-30s.
(the limb bud experiments and also the inner ear experiments, and many others.)
&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&
The Apical Ectodermal Ridge (AER) is a thickening of the epidermis
that forms along the outer rim of the limb bud
(of bird, mammal, reptile,
frog and fish embryos - but NOT on limb buds of salamanders!)
In frog embryos, the AER is comparatively small and indistinct,
and for many years, everyone believed that frogs didn't have an AER.
But more careful studies found them.
The scientific meeting at which this
evidence was first presented was in Wales in 1971, & I was in the audience!
Surgical removal of the AER causes failure of the distal structures to develop.
Cut the AER off early, then no elbow, forearm, wrist or hand.
Cut the AER off later, then no wrist & hand.
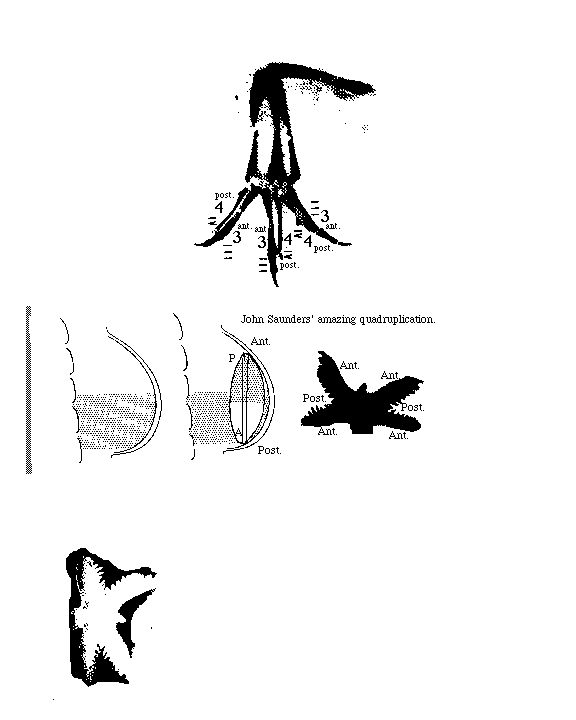
This was discovered by Prof. John Saunders, who taught for many years
at the State University of New York in Albany,
and who visited this department
some years ago, and is one of the most stimulating scientists I have ever met.

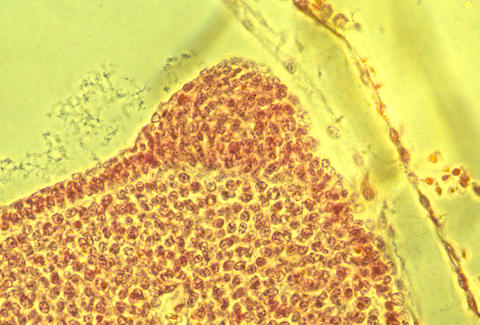
apical epidermal ridge of a chick embryo
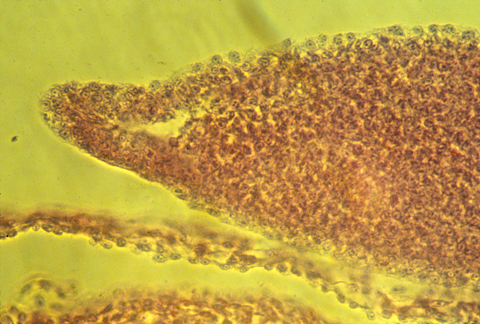
apical epidermal ridge of a mouse embryo
In sections of bird AERs, the epithelial cells seem to be pinched
together at their basal ends,
and the ridge is as much a "pucker"
as a thickening.
Sections through mouse embryo AERs show a different geometry.
It is definitely a thickening,
but with some indication of contraction at
Both basal and apical surfaces.
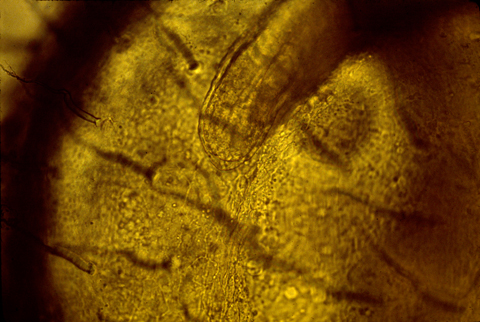
Here is a photograph of an oblique, or slanted section through a chicken AER.
The developing tail fins of fish have a similar thickened ridge.
Grafting an extra AER can cause a double wing to form.
A mutation in chickens causes double AERs and double wings!
But the AER doesn't itself become any these structures.
It somehow signals to them to form.
There has been much research and several theories on this subject.
Fibroblast growth factor 10 seems to be the key signal molecule.
FGF10 can replace the effect of the AER;
If you cut off the AER, & put a bead coated with FGF in its place,
then the distal limb structures will form more or less normally.
(If anybody has done this with snake embryos, I haven't heard about it!
Probably, snakes still have the genes for legs, and maybe the signal receptors.)
Legless lizards and legless salamanders might also be subject to such experiments.
Another interesting fact is that salamanders (especially newts) are the
only kinds of vertebrates
that can regenerate cut off legs, and also are the only kind of vertebrate that don't form an AER.
However, during regeneration of salamander legs, an epithelial thickening
is formed at the tip
of the regenerating limb stump!
This thickening is not elongated, however.
I suggest to you that some big conceptual breakthrough is waiting for you to discover!
&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&&
Limb regeneration in salamanders
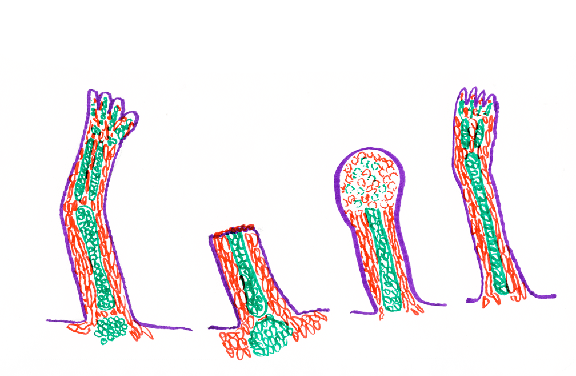
I) Newts and many other kinds of salamanders can regenerate their legs.
No other vertebrates can do this, except partial regeneration of limbs in frog tadpoles before metamorphosis.
Humans can sometimes regenerate the last digit of fingers and toes, IF the wound is NOT stitched up (which is standard practice).
Regenerating newt legs "re-grow" all the bones (i.e. cartilages), muscles, tendons, blood vessels, connective tissue and skin.
In the stump of the cut limb, cartilage, muscle and other cell type become indistinguishable, "dedifferentiating" back to what looks like their early embryonic state, forming a mass of cells called a blastema, in which rapid cell growth and mitosis replaces the lost cells.
Skeletal muscle cells, which are syncytial, subdivide back to individual cells before resuming mitosis.
It would be interesting to know whether this subdivision of multi-nucleate muscle cells
has the same contractile-ring mechanism as the ordinary cytokinesis that follow mitosis.
The myoblasts then undergo many cycles of mitotic cell divisions, and fuse back together.
II) Grafting of cartilage and muscle labeled with radioactive labeled DNA, and also the use of genetic markers like nucleolus number, consistently show that each cell remains "loyal" to their original differentiated cell type.
In other words the dividing cells in the blastema that had been cartilage cells
re-differentiate only into cartilage,
& the descendants of muscle cells only become muscle.
Most experimenters had not expected this, which make the results even more persuasive.
It also contradicts expectations that regeneration should have the same basic mechanism as the original formation of limbs, except if the original development was by sorting out of cells whose differentiation had already been decided, which nobody considers possible.
Limb regeneration is not thought of as a special case of sorting out by differentiated cells, I guess because there is no deliberate dissociation into single or randomly-mixed cells.
Nevertheless, consider that cartilage cells from which the humerus had previously been constructed separate into what seem to be undifferentiated cells, and then re-differentiate as the radius, ulna digits, wrist bones, as well as replacements for the lost parts of the humerus.
Furthermore, consider that the many muscles of the regenerated leg are made of cells that had been parts of different muscles of the upper limb.
Likewise: skin cells, blood vessels, connective tissue & nerves.
All are replaced by descendants of the same kinds of the cells in the stump of the cut limb.
III) Salamanders are the only vertebrates whose limb buds do not form an apical ectodermal ridge.
However, during regeneration of salamander, the stumps develop an epithelial thickening which is well-formed, but not elongated in the A-P axis (unlike the AER)
I can't figure out how to interpret this combinations of odd facts, but it must mean something.
IV) If you cut off just the "hand" part of a newt leg, then just that part will regenerate.
If you cut the leg off at the elbow or knee, then just the missing part will regenerate.
If you cut off the whole leg, than all the parts of will regenerate.
BUT: If you cut off just the "hand" part, and then paint the cut surface with retinoic acid, then a whole new leg will regenerate, starting over at the shoulder, and forming an extra elbow, etc.
AND: Newts can regenerate their tails.
BUT if you cut off the tail, and paint the cut surface with retinoic acid, guess what regenerates instead!
The more of a newt leg you cut off, the faster tissues regenerate; So that the total time to completion of regeneration is about the same when you cut off the whole leg as when you cut off just the tip.