Lecture notes for Friday, April 21, 2017
Cancer
Abnormalities of Cancer Cells
1) Glucose uptake is much higher than normal in most cancer cells.Related to this is an abnormally (very) large secretion of lactic acid, and abnormally low amounts of ATP production by mitochondria. This set of inter-related abnormalities was discovered around 1930 by Warburg, who had won the Nobel Prize for previous discoveries, and is one of two (unrelated) phenomena both called "The Warburg Effect". Excess glucose uptake into cancer cells is the reason why PET scans can detect cancer, and reliably distinguish cancer cells from normal cells. I have had several PET scans myself, and they accurately mapped the tumors. They cost about three thousand dollars per scan.
The biochemical cause of these phenomena remains unknown, and very little research has been done on it, because it doesn't fit in with oncogene research. The best English language textbook on cancer biology doesn't even mention the Warburg effect. Almost no research has been done about how to kill cancer cells based on either their anaerobic metabolism, or the excess uptake of glucose. (Despite very large amounts of research on how to use the phenomenon to map tumors).
2) Magnetic Resonance Images of cancer cells are visibly different than normal tissues.
This was the subject of the first research paper about MRI.
Nobody ever discovered the reason why cancer cells' MRI images are abnormal.
Almost no research has been done on the subject.
3) The acto-myosin cytoskeleton of cancer cells is disorganized, and they exert much weaker traction forces as they crawl.
4) Cancer cells can crawl onto less adhesive substrata,
from more adhesive substrata. This is a loss of haptotaxis.
It was discovered by Prof. Barbara Danowski, as part of her PhD research
in this department. She invented the experiment herself. She then did
important research at the University of Pennsylvania, and is now
Professor and Chair of Biology at Union College in New York.
I had never considered the possibility, until she proved it.
5) Defective cell-cycle checkpoint controls.
For example, cancer cells tend to go ahead and copy DNA, even if it is damaged.
Normal cells would delay starting to copy their DNA until
damage to it has been repaired.
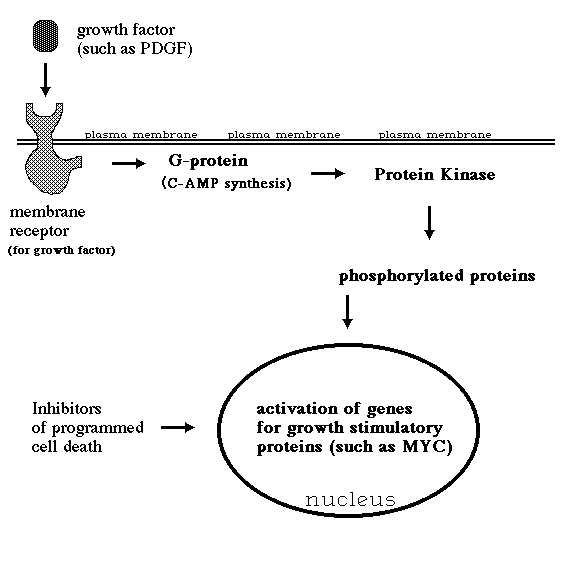
6) Excessive inhibition of apoptosis.
In particular, 95% of cases of the "follicular" kind of Non-Hodgkin's lymphoma
are caused by a breakage and incorrect re-joining of the 14th chromosome
and the 18th chromosome, in such a way that the promoter region of the
antibody heavy chain gene is next to a gene named bcl-2. This stands for
b-cell lymphoma two, because it was the second lymphoma-causing gene to be
discovered by looking for chromosome translocations in cancerous lymphocytes
of human patients. Nematodes have a gene almost identical to human bcl-2.
It is needed to inhibit programmed cell death, both in vertebrates and in
nematodes (and probably all animals). Experimenters have even spliced the
human DNA sequence for bcl-2 into nematodes, in place of the nematode's own
gene. The human gene works fine in nematode embryonic development.
When a human b-lymphocyte has this particular chromosome translocation, it makes bcl-2 protein instead of antibody, and can't undergo programmed cell death. Although such lymphocytes don't grow or divide any faster than normal lymphocytes, they do accumulate without limit. Eventually, they displace your normal lymphocytes and fill up your bone marrow, until you become anemic, can't make antibodies etc. and die.
Ironically, the treatment is often a chemical designed to kill fast-growing cells by damaging their DNA. It was assumed the cancerous lymphocytes must be growing too fast, and that cross-linking their DNA would selectively kill the fastest growing lymphocytes.(That, itself, isn't very logical. Why should it kill a cell to prevent it from doing something abnormal? If they grew slower than normal, would you expect to be able to kill them by speeding them up?)
Consider the following hypothesis about how chemotherapy really works:
When DNA is damaged by radiation or a drug like cyclophosphamide,
non-cancerous cells detect the damage and slow down their growth,
halting at a check-point until the damaged DNA has been fixed.
The cancerous cells, however, because their check-point controls are broken,
continue to copy DNA and undergo mitosis. This failure to stop is really
what kills them. This is sort of the opposite of what had been assumed.
Cancer cells die because they can't stop, not because they grow too fast.
Any time a drug works, there must be some reason; but the true reason may
be very different than what the drug was designed to do.
7) Lack of anchorage dependence, the ability of cells to survive
and continue dividing without being spread out on a solid substratum.
Cancer cells can grow and survive suspended in a gel. Non-cancerous cells
will undergo programmed cell death if you culture them on an area of substratum
that is smaller that the area they normally occupy when attached to a Petri
dish. Nicholas Maroudas and Donald Ingber have done the best research on this.
8) Increased secretion of proteolytic enzymes
9) Loss of differentiated characteristics ("tumor progression")
--------------------------------------------------------------------------------------------------------------------
Causes of Cancer
The great majority (>95%) of human cancers are caused by somatic mutations in a few specific genes (called "Oncogenes").
In many other species, large fractions of cancers are caused by communicable viruses. (Examples include cats and turkeys)
This department gives a very good course specifically about oncogenes and how they cause cancer.
Several sexually transmitted papilloma viruses cause cervical cancer in women. A vaccine has been developed which inhibits infection by these cancer-causing papilloma viruses.
---------------------------------------------------------------------------------------------------------
Cancer cells retain many of the properties of whichever differentiated cell type they began from:
